Coordination of cell signaling, trafficking and proteostasis in health and disease
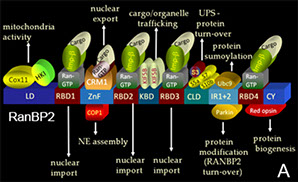
Background. The Ran-binding protein 2 (RanBP2) is a large 358 kDa scaffold protein, which is comprised of a number of distinct structural modules. Structure-function studies of RanBP2 had led to the identification of a number of partners with apparent disparate functions and specific binding activities toward selective domains of RanBP2 (A). Studies from multiple laboratories and ours suppport that RanBP2 plays critical cell-stage and cell-context-dependent biological or pathophysiological roles in nucleocytoplasmic and microtubule-based intracellular trafficking, nuclear envelope breakdown and mitosis, modulation of protein homeostasis by the ubiquitin-proteasome system, mitochondrial function and trafficking, regulation of protein-protein interactions and localizations by SUMOylation, negative regulation of cAMP signaling and gene-environment interactions modulating glucose and lipid metabolism. Although complex and not well understood, RanBP2’s pleiotropic and cell context-dependent properties reflect most likely combinatorial functions of (1) the association of distinct domains of RanBP2 with proteins presenting distinct functional properties, (2) multifunctional properties associated to single domains of RanBP2, (3) cross-talk between adjacent domains of RanBP2 and its partners or (4) a combination of these features. Human semi-dominant mutations affecting selective domains of RANBP2 have a severe impact in the central nervous system and cause familial necrotic encephalopathies. Further, RanBP2 is essential for organism survival and RanBP2 insufficiency plays a critical role in oncogenesis, neuroprotection and a variety of gene-environment responses.
Current projects. We are engaged in a number of synergizing topics and interdisciplinary approaches that range from single molecule level analyses of the interplay between RanBP2, kinesin-1 and other partners, cellular assays to uncover biological effects of the interaction of domains of RanBP2 with its partners, to defining physiological and pathological correlates between overall or selective biological functions of RanBP2 in distinct cell types of genetic mouse models of RanBP2 and molecular functions and mechanisms linked to RanBP2, its domains or partners thereof.
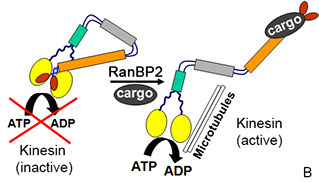
1. A theme of our work has been to uncover kinetic mechanisms of regulation of kinesin-1 activities (B). RanBP2 was the first cargo shown to activate a kinesin in a minimal in vitro system of purified components. Current studies aim at establishing direct corollaries between single molecule activity outcomes and the regulation of motor-driven subcellular processes, such as mitochondrial motility and function, by domains or subdomains of RanBP2 or its partners. These studies are producing novel mechanistic insights into several competitive and cooperative modalities of regulation of kinesin motor activities, RanBP2 functions, mitochondrial dynamics or a combination of these that may be amenable to distinct therapeutic interventions in a number of disorders of various etiologies.
2. Another topic of study is the regulation of proteostasis by RanBP2 and some of its partners. Our laboratory found that at least two distinct domains of RanBP2 control the proteostasis of selective substrates expressed by distinct tissues or cell types. The modulation of proteostasis by RanBP2 is achieved by chaperone or foldase activities harbored by selective domains of Ranbp2 that directly or indirectly affect unique functions of the ubiquitin-proteasome system (UPS). Current studies are uncovering proteostasis mechanisms and targets controlled by Ranbp2 and the implications of these processes on substrates underpinning a variety of pathogenic processes in distinct cell-types or tissues. These studies will pave the way the development of novel pharmacological strategies to harness and unveil novel therapeutic potentials of Ranbp2 and its partners in multiple disease states.
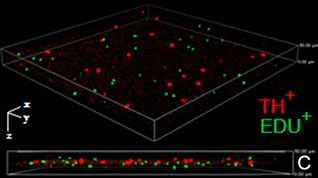
3. A third theme of our work has been discovering and defining gene-environment responses mediated by RanBP2 upon disease stressors. Currently, we are investigating a number of extrinsic and intrinsic disease stressors, such as phototoxicity, Parkinsonian neurotoxic insults and disease mutations. For example, our data support that an overall deficits in RanBP2 play antagonizing roles in neuroprotection between distinct neuronal or supporting cell types, such as dopaminergic and photoreceptor neurons and glial cells, against distinct disease stressors (C). These studies aim also at defining the activation of extrinsic and intrinsic disease pathways regulated by RanBP2 and its partners and that are likely to offer new therapeutic prospects.
4. A hallmark manifestation of insufficiency of RanBP2 is a change in the cellular/tissue metabolic profile, in particular of glucose and lipid metabolites (e.g. free fatty acids and cholesterol). Some of these metabolic changes appear to confer age-dependent neuroprotection to selective neurons with high susceptibility to oxidative stress, whereas other cells lack such protection. Hence, efforts are underway to define the molecular bases of regulation of distinct metabolic pathways by RanBP2 and some of its partners and their roles in neuronal cell death and neuroprotection.
Duke University Medical Center, 2351 Erwin Road, DUEC 3802, Durham, NC 27710, USA